Table Of Content
By the way, the table above also shows the traceability between user needs and test cases. This trace matrix provides part of the V&V evidence that the FDA requires. Join 200,000+ other medical device professionals outperforming their peers.
How do you manage design verification activities across different teams and stakeholders?
Design input is any physical and performance requirement that is used as the basis for designing purpose. Design output is the result of each design phase and at the end of total design effort. Validation is concerned with demonstrating the consistency and completeness of design with respect to the user needs.
Manage all your verification and validation activities with a dedicated design control software
Accelerate chip-design verification process by running Siemens EDA Calibre on AWS Amazon Web Services - AWS Blog
Accelerate chip-design verification process by running Siemens EDA Calibre on AWS Amazon Web Services.
Posted: Mon, 26 Feb 2024 08:00:00 GMT [source]
You’ll be able to easily navigate between test reports and protocols to your design control verifications and validations, while also seeing related design inputs, risk controls, and components. The sooner you begin thinking about how you’ll verify these design inputs, the smoother the actual verification process will go. In fact, you should be considering how you’ll verify your design inputs while you’re defining them. If you don’t have plans in place for verification and validation, you’ll end up wasting an enormous amount of time on these steps, and you may miss key activities that could hold up your regulatory timeline. As with design inputs, be sure that your user needs are clearly defined at the beginning of the design and development process. For example, if one of the user needs is that “the device needs to be safe,” how will you validate that?
Product
Here we’ll explain what the two activities are, the difference between them, plus share tips for getting the most out of your efforts. You can always leverage previous lessons, experiences, and data, and the types of design changes you’ve made will determine how much verification and validation you’ll need to redo. But there simply can’t be a point in the process where you’re unsure whether you’ve made the right device and that it’s working correctly. Design verification and design validation are two essential stages in design controls. It’s easy to confuse the two because they both involve checking an outcome against your previous specifications.
Advantages of design validation and verification
Take time to define how you want tools to support your team before you choose. You’ll also want to consider the required resources, manpower, and tools for successful verification. Includes final report, with test results and traceability, ready for regulatory review. Includes testing of production equivalent units under real-use conditions. We’ve identified our user needs; now let’s identify what the device has to do and how it has to do it.
Common Pitfalls in Design Validation
No matter when in the product lifecycle you make the change, you can’t skip verification and validation. Even in mid- and post-market stages, before and after product updates, you have to be ready to verify and validate. Planning and executing design verification and validation requires a deep understanding of the relationships between design controls. And if you’re using a paper-based QMS to keep track of all your work, it’s impossible to step back and see the full picture. This will often require more testing that will be used on production models. To ensure that all requirements are met, a full set of measurement and tests is done on the validation unit.
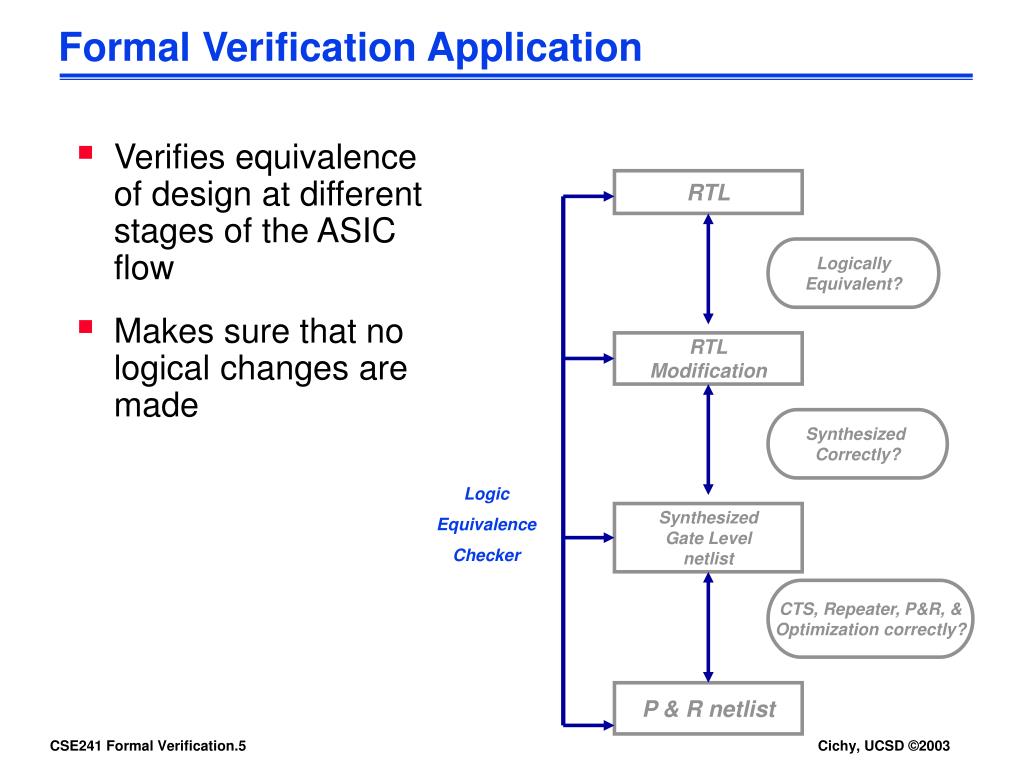
Design verification is a critical step in the design control process, where you ensure that your design outputs meet the design inputs and the intended use of your product. However, managing design verification activities across different teams and stakeholders can be challenging, especially if you have complex, multidisciplinary, or distributed projects. In this article, you will learn some best practices and tips to plan, execute, and document your design verification activities effectively and efficiently. By conducting design verification activities across multiple layers of the design process, organizations can effectively mitigate risks and ensure that the final design meets the desired objectives and requirements. This comprehensive approach to design verification helps identify and rectify any potential issues or shortcomings early on, leading to a more robust and reliable end product. By adhering to the FDA’s requirements for design verification and validation, medical device manufacturers can ensure the development of safe, effective, and compliant devices.
Reporting
He also served in various project management roles and has led global, cross-functional development teams for a wide variety of programs. During this time, he developed several award-winning and patented product designs. Greg holds bachelor and master of science degrees in mechanical engineering from the Georgia Institute of Technology.
AV Verification, Validation Demands Merging of Real, Virtual Worlds - Ward's Auto
AV Verification, Validation Demands Merging of Real, Virtual Worlds.
Posted: Tue, 19 Mar 2024 07:00:00 GMT [source]
Like any tool, it takes training and practice to gain the full benefit of the DVP&R. Once fully implemented into your company’s culture you will soon begin to realize the benefits. If you develop products — medical devices, particularly — then you’ve heard the terms and design verification and validation (also called V&V).
Conduct tests according to the plans you made in the preparation phase. Stay ahead 🪄 by getting introduced to new handpicked 🤖 AI tools 1x a week. This presentation starts with the introduction of our FPGA-based SoC control system DUT and testbench, then discusses advantages and disadvantages of using Vivado and Questa Core for verification. Learn how Allegro MicroSystems leveraged AI-powered Solido Design Environment and Analog FastSPICE to accelerate PMIC verification, resulting in an overall 5X reduction in verification time.
These are both essential aspects of ensuring the medical device can be used safely and effectively. ISO 9001 Design Verification and Design Validation are two steps that are distinctly different, and important in a good design process. Verification is used to make sure that the design has addressed every requirement, while validation is used to prove that the design can meet the requirements set out for it. You need to adapt the design verification and validation process to the needs of different industries. Design validation involves testing a product to meet the users’ precise requirements.
Verification – confirmation by examination and provision of objective evidence that specified requirements have been fulfilled. Traceability – The ability to ensure that requirements outlined in the specifications have been tested. Qualification – A testing protocol designating that a system meets a defined collection of requirements. Test plan, test cases, test execution records, and test results should be documented and maintained as a part of design records.
Product requirements (often included in a product requirements document) for our user need might look like below. Basically, in design validation, we need to demonstrate that the product meets the user’s needs. By the end of the process, you should have a list of design inputs and verification tests for each that will demonstrate that the device does what you intend it to do. More often than not, design verification involves suites of tests and trials. However, there are other acceptable verification activities, such as inspection and analysis (for a more extensive list of activities, refer to page 30 of the FDA Design Control Guidance). In this episode of the Global Medical Device podcast, we delve into everything related to design verification and validation, which is often called V&V.

No comments:
Post a Comment